Alurar rigakafin kyanda
|
essential medicine (en) | |
 | |
| Bayanai | |
| Ƙaramin ɓangare na |
vaccine (en) |
| Vaccine for (en) | baƙondoro |
Alurar rigakafin kyanda na kare kariya daga kamuwa da cutar kyanda . [1] Kusan duk waɗanda ba su haɓaka rigakafi ba bayan kashi ɗaya suna haɓaka ta bayan kashi na biyu. [1] Lokacin da adadin allurar rigakafi a cikin jama'a ya fi kashi 92%, barkewar cutar kyanda yawanci ba ya faruwa; duk da haka, suna iya sake faruwa idan adadin rigakafin ya ragu. [1] Tasirin maganin yana ɗaukar shekaru masu yawa. [1] Ba a sani ba idan ya zama ƙasa da tasiri akan lokaci. [1] Hakanan maganin na iya yin kariya daga cutar kyanda idan aka ba shi cikin kwanaki biyu bayan kamuwa da cutar kyanda. [2] [1] [3] [4]
Gabaɗaya maganin yana da lafiya, har ma ga waɗanda suka kamu da cutar kanjamau . [1] [5] Yawancin yara ba sa fuskantar wani illa; [6] wadanda ke faruwa yawanci suna da laushi, irin su zazzabi, kurji, zafi a wurin allura, da taurin haɗin gwiwa; kuma gajere ne. [1] [6] An rubuta anaphylaxis a cikin kusan lokuta 3.5-10 a kowace allurai miliyan. [1] Adadin ciwon Guillain-Barré, Autism da cututtukan hanji mai kumburi ba sa alama an ƙara su ta hanyar rigakafin kyanda. [1] [7]
Ana samun maganin alurar riga kafi da kanta kuma a cikin haɗuwa kamar maganin MMR (haɗuwa tare da rigakafin rubella da maganin mumps ) [1] ko maganin MMRV (haɗin MMR tare da maganin kaji ).[8] [9] [10] Maganin cutar kyanda yana da tasiri daidai gwargwado don hana cutar kyanda a cikin dukkan hanyoyin da aka tsara, amma illar illa sun bambanta ga haɗuwa daban-daban. [1] [11] Hukumar Lafiya ta Duniya (WHO) ta ba da shawarar a ba da allurar rigakafin cutar kyanda a cikin watanni tara a sassan duniya da cutar ta yadu, ko kuma a watanni goma sha biyu inda cutar ba ta da yawa. [12] [1] Maganin cutar kyanda ya dogara ne akan nau'in cutar kyanda mai rai amma rauni . [1] Yana zuwa ne a matsayin busasshen foda wanda ake hadawa da wani ruwa na musamman kafin a yi masa allura ko dai a karkashin fata ko a cikin tsoka. [1] Tabbatar da cewa maganin yana da tasiri ana iya tantance shi ta gwajin jini. [1]
An fara gabatar da rigakafin cutar kyanda a 1963. A waccan shekarar, cutar kyanda ta Edmonston-B ta zama maganin rigakafi ta John Enders da abokan aiki kuma sun ba da lasisi a Amurka. [13] [14] A cikin 1968, Maurice Hilleman da abokan aiki sun samar da ingantaccen rigakafin cutar kyanda har ma da rauni, kuma an fara rarraba shi, ya zama kawai maganin cutar kyanda da aka yi amfani da shi a Amurka tun 1968. [15] [13] Kimanin kashi 86% na yara a duniya sun sami aƙalla kashi ɗaya na maganin alurar riga kafi kamar na 2018. [16] A cikin 2021, aƙalla ƙasashe 183 sun ba da allurai biyu a cikin jadawalin rigakafi na yau da kullun. [17] Yana cikin Jerin Mahimman Magunguna na Hukumar Lafiya ta Duniya . [18] Kamar yadda barkewar cutar cikin sauƙi a cikin al'ummomin da ba a yi wa allurar rigakafi ba, ana ganin rashin yaɗuwar cututtuka azaman gwajin isassun allurar rigakafi a tsakanin al'umma. [19][20]
Tasiri
[gyara sashe | gyara masomin]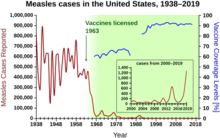


Kashi ɗaya yana da kusan kashi 93% yana tasiri yayin da allurai biyu na allurar rigakafi kusan kashi 97% ke da tasiri wajen hana cutar kyanda. [5] Kafin yin amfani da rigakafin cutar kyanda, cutar kyanda ta zama ruwan dare wanda har ana daukar kamuwa da cuta "kamar makawa kamar mutuwa da haraji." [21] A cikin Amurka, an ba da rahoton bullar cutar kyanda daga 3 zuwa miliyan 4 tare da mutuwar 400 zuwa 500 zuwa dubun dubbai a kowace shekara bayan gabatar da allurar rigakafin kyanda guda biyu a cikin 1963 (dukkanin da ba a kunna ba da kuma maganin rigakafi mai rai (Edmonston B iri). ) an ba su lasisi don amfani, duba ginshiƙi a dama). [5] [22] Ƙara yawan maganin alurar riga kafi bayan barkewar cutar a cikin 1971 da 1977 ya kawo wannan ga dubban lokuta a kowace shekara a cikin 1980s. Barkewar cutar kusan 30,000 a cikin 1990 ya haifar da sabunta turawa don yin rigakafi da ƙari na rigakafi na biyu zuwa jadawalin da aka ba da shawarar. Ba a sami rahoton bullar cutar sama da 220 a kowace shekara daga 1997 zuwa 2013 ba, kuma an yi imanin cewa cutar ba ta da yawa a Amurka. [23] [24] [25] A cikin 2014, an sami rahoton shari'o'i 667. [26]
Amfanin allurar rigakafin kyanda a cikin rigakafin cututtuka, nakasa, da mutuwa an rubuta su sosai. A cikin shekaru 20 na farko na samun lasisi a Amurka, rigakafin cutar kyanda ya hana kimanin mutane miliyan 52 na cutar, lokuta 17,400 na nakasa hankali, da kuma mutuwar 5,200. [27] Daga 1999 zuwa 2004 dabarar da WHO da UNICEF suka jagoranta ta haifar da ingantuwar rigakafin cutar kyanda wanda ya kawar da kiyasin mutuwar cutar kyanda miliyan 1.4 a duniya. [28] Maganin rigakafin cutar kyanda ya haifar da kusan kawar da cutar a Amurka da sauran kasashen da suka ci gaba. [29] Yayin da aka yi maganin alurar riga kafi da kwayar cuta mai rai wanda zai iya haifar da illa, waɗannan sun yi ƙanƙanta kuma ba su da tsanani fiye da rashin lafiya da mutuwar cutar kyanda da kanta; illa masu kama daga rashes zuwa, da wuya, jujjuyawa, suna faruwa a cikin ƙaramin adadin masu karɓa.[30]
Alurar rigakafin kyanda ya kawar da mutuwar mutane miliyan 57 tsakanin 2000 zuwa 2022, kamar yadda rahoton Hukumar Lafiya ta Duniya ya nuna. [31]
Cutar kyanda ta zama ruwan dare gama duniya. Ko da yake an sanar da kawar da ita daga Amurka a shekara ta 2000, ana buƙatar yawan allurar rigakafi da kyakkyawar sadarwa tare da waɗanda suka ƙi yin rigakafi don hana barkewar cutar da kuma ci gaba da kawar da cutar kyanda. [32] Daga cikin kararraki 66 na cutar kyanda da aka ruwaito a Amurka a cikin 2005, sama da rabi an danganta su ga wani matashin da ba a yi masa allurar rigakafi ba wanda ya kamu da cutar yayin ziyarar Romania. [33] Wannan mutumin ya koma wata al'umma tare da yara da yawa da ba a yi musu rigakafi ba. Sakamakon bullar cutar ta kama mutane 34, galibi yara kuma kusan dukkansu ba a yi musu allurar ba; uku daga cikinsu suna asibiti. Amsar lafiyar jama'a ta buƙaci yin kusan kiran waya 5,000 a zaman wani ɓangare na tuntuɓar tuntuɓar, tsarawa da yin gwaji kamar yadda ake buƙata, da kuma shirya rigakafin gaggawa ga mutanen da ke cikin haɗarin da suka yi hulɗa da wannan mutumin. [32] Masu biyan haraji da ƙungiyoyin kiwon lafiya na gida wataƙila sun biya sama da dalar Amurka 167,000 a cikin farashi kai tsaye don ɗaukar wannan barkewar. [32] An kawar da wata babbar annoba saboda yawan allurar rigakafi a cikin al'ummomin da ke kewaye. [32]
Alurar rigakafin ba ta da takamaiman tasiri kamar hana kamuwa da cututtukan numfashi, wanda zai iya girma fiye da na rigakafin kyanda kaɗai. [34] Waɗannan fa'idodin sun fi girma idan an ba da maganin kafin shekara ɗaya. [35] Alurar riga kafi ya haifar da mummunan sakamako a cikin 'yan mata, saboda haka Hukumar Lafiya ta Duniya ba ta ba da shawarar ba. [36]
Amsar rigakafin rigakafi ga rigakafin kyanda na iya lalacewa ta kasancewar cututtukan cututtuka irin su helminthiasis . [37]
Jadawalin
[gyara sashe | gyara masomin]Hukumar Lafiya ta Duniya (WHO) ta ba da shawarar allurai biyu na alluran rigakafi ga duk yara. [1] A cikin ƙasashe masu haɗarin kamuwa da cuta ya kamata a ba da kashi na farko a kusan watanni tara. [1] In ba haka ba za a iya ba da shi a cikin watanni goma sha biyu. [1] Ya kamata a ba da kashi na biyu aƙalla wata ɗaya bayan kashi na farko. [1] Ana yin wannan sau da yawa a cikin shekaru 15 zuwa watanni 18. [1] Bayan kashi daya a cikin watanni tara 85% na rigakafi, yayin da kashi a watanni goma sha biyu yana haifar da kashi 95% na rigakafi.
A cikin Amurka, Cibiyar Kula da Cututtuka da Cututtuka (CDC) ta ba da shawarar cewa yara masu shekaru shida zuwa watanni goma sha ɗaya da ke balaguro zuwa Amurka su sami kashi na farko na rigakafin MMR kafin tashi [38] sannan su sami ƙarin allurai biyu; daya a watanni 12-15 (watanni 12 ga yara a wuraren da ke da haɗari) kuma na biyu a farkon makonni huɗu bayan haka. [39] In ba haka ba ana ba da kashi na farko a cikin watanni 12-15 kuma na biyu a shekaru 4-6.[40]
A Burtaniya, Shawarar Ma'aikatar Kiwon Lafiya ta Kasa (NHS) ita ce kashi na farko a kusan watanni 13 da haihuwa kuma na biyu a shekara uku da wata hudu. [41] [42]
A Kanada, Lafiyar Kanada ta ba da shawarar cewa yaran da ke tafiya a wajen Arewacin Amurka su sami rigakafin MMR idan sun kai watanni shida zuwa 12. Koyaya, bayan yaron ya cika watanni 12 yakamata su sami ƙarin allurai biyu don tabbatar da kariya mai dorewa. [43]
Tasiri mara kyau
[gyara sashe | gyara masomin]Mummunan illa da ke hade da maganin rigakafi na MMR sun hada da zazzabi, rash, ciwon wurin allura da kuma, a lokuta da yawa, launin ja ko shunayya a kan fata da aka sani da thrombocytopenic purpura, ko ciwon da ke da alaka da zazzaɓi ( zazzaɓi ). [44] [45]
Yawancin karatu ba su sami dangantaka tsakanin rigakafin MMR da Autism ba.[45][46][47]
Contraindications
[gyara sashe | gyara masomin]Ba shi da kyau ga wasu mutane su karɓi maganin kyanda ko MMR, gami da lokuta na:
- Ciki: Kada a ba mata masu ciki allurar MMR da abubuwan da ke cikinsa. [48] Matan da suka kai shekarun haihuwa su tuntubi likitansu game da yin allurar riga-kafi kafin su dauki ciki. [10]
- Yaran da ke kamuwa da cutar HIV, waɗanda za su iya samun rigakafin cutar kyanda idan adadin CD4+ lymphocyte ɗin su ya fi 15%.[49]
- Rashin raunin garkuwar jiki saboda HIV/AIDS ko wasu jiyya [10]
- Samun iyaye ko ɗan'uwa tare da tarihin matsalolin rigakafi [10]
- Yanayin da ke sa majiyyaci rauni ko zubar jini cikin sauƙi [10]
- Karan jini ko samfuran jini na baya-bayan nan [10]
- Tarin fuka [10]
- Karbar wasu alluran rigakafi a cikin makonni 4 da suka gabata [10]
- Matsakaici ko rashin lafiya mai tsanani. Koyaya, rashin lafiya mai sauƙi (misali, mura na gama gari) yawanci ba a hana shi ba. [10]
Tarihi
[gyara sashe | gyara masomin]
John Franklin Enders, wanda ya raba lambar yabo ta Nobel a fannin likitanci na 1954 don aiki kan cutar shan inna, ya aika Thomas C. Peebles zuwa makarantar Fay a Massachusetts, inda aka fara barkewar cutar kyanda; Peebles ya iya ware kwayar cutar daga samfurin jini da kuma swabs na makogwaro, kuma daga baya ya sami damar noma kwayar cutar tare da nuna cewa ana iya yada cutar ga birai da kayan da ya tattara. [29] Enders ya sami damar yin amfani da kwayar cutar da aka noma don samar da rigakafin cutar kyanda a cikin 1963 ta hanyar attenuation ta hanyar al'adar kaji fibroblasts na kayan da Peebles ke ware. [50] [51]
A ƙarshen 1950s da farkon 1960, kusan ninki biyu na yara sun mutu daga cutar kyanda kamar na polio. [52] Alurar rigakafin Enders ta samo asali ne daga nau'in cutar kyanda mai rai na Edmonston, wanda aka sanya wa suna David Edmonston mai shekaru 11, dalibin Fay wanda Peebles ya dauki al'adar da ta haifar da noman cutar. [53]
A tsakiyar karni na 20, cutar kyanda ta yi kamari a yammacin Afirka, inda yawan mace-macen yara ya kai kashi 50 cikin 100 kafin su cika shekaru biyar, kuma yaran sun kamu da nau'in kurji da sauran alamomin da aka saba gani kafin 1900 a Ingila da wasu kasashe. , Najeriya; [54] idan za a iya zarge shi da cin zarafin al'ummar Najeriya, Morley ya hada 'ya'yansa hudu a cikin binciken. Sakamakon karfafa gwiwa ya kai ga yin nazari na biyu na kimanin yara 450 a kauyen da kuma asibitin Wesley Guild da ke Ilesha.[ana buƙatar hujja]
Bayan wata annoba, an gudanar da gwaji mafi girma a cikin Satumba da Oktoba 1962, a cikin New York City tare da taimakon WHO: Yara 131 sun sami rai mai rai na Enders-attenuated Edmonston B da gamma globulin, yara 130 sun sami "ƙarin ragewa" rigakafin. ba tare da gamma globulin ba, kuma yara 173 sun zama batutuwa masu sarrafawa na ƙungiyoyin biyu. Kamar yadda kuma aka nuna a gwajin da aka yi a Najeriya, gwajin ya tabbatar da cewa maganin “wanda aka kara ragewa” ya fi allurar Edmonston B, kuma ya haifar da karancin zazzabi da gudawa. Yara 2,000 a yankin an yi musu allurar rigakafin da aka kara ragewa. [55][56]
Maurice Hilleman a Merck & Co., majagaba a cikin ci gaban alurar riga kafi, ya samar da ingantacciyar sigar rigakafin cutar kyanda a cikin 1968 kuma daga baya rigakafin MMR a 1971, wanda ke yin rigakafin cutar kyanda, mumps da rubella a cikin harbi guda tare da mai haɓakawa. . [13] Ana kiran nau'i ɗaya "Attenuvax". [57] Bangaren kyanda na maganin rigakafin MMR yana amfani da Attenuvax, [58] wanda aka girma a cikin al'adar mahaifar kaji ta amfani da nau'in Edmonston da aka rage ta Enders. [58] Biye da shawarwarin ACIP, Merck ya yanke shawarar kada ya ci gaba da samar da Attenuvax a matsayin maganin rigakafi a kan 21 Oktoba 2009. [59]
Wani bincike na 2022 a cikin Mujallar Tattalin Arziki ta Amurka ya gano cewa ɗaukar rigakafin cutar kyanda ya haifar da haɓakar samun kuɗin shiga na kashi 1.1 cikin ɗari da kuma tasiri mai kyau akan aikin yi saboda yawan samarwa da waɗanda aka yiwa alurar riga kafi. [60]
Nau'ukan
[gyara sashe | gyara masomin]
Ba safai ake ba da kyanda a matsayin mutum na alurar riga kafi kuma ana ba da shi a hade tare da rubella, mumps, ko varicella (kaza) alurar riga kafi. [1] A ƙasa akwai jerin alluran rigakafin da ke ɗauke da kyanda:
- Alurar rigakafin kyanda (alurar riga kafi)
- Cutar kyanda da rubella hade maganin alurar riga kafi ( MR )
- Mumps, kyanda da rubella hade maganin rigakafi ( MMR alurar riga kafi ) [58] [61] [62]
- Mumps, kyanda, rubella da varicella hade maganin alurar riga kafi (MMRV ) [9]
Al'umma da al'adu
[gyara sashe | gyara masomin]Yawancin tsare-tsaren inshora na kiwon lafiya a Amurka suna biyan kuɗin alluran rigakafi, kuma Shirin Alurar rigakafi na Yara na iya taimakawa waɗanda ba su da ɗaukar hoto. [63] Dokar jiha tana buƙatar yin alluran rigakafi ga yaran makaranta, amma tana ba da keɓe don dalilai na likita wasu lokuta saboda dalilai na addini ko falsafa. [64] Duk jihohi hamsin suna buƙatar allurai biyu na rigakafin MMR a shekarun da suka dace. [65] Rarraba allurar rigakafi daban-daban a cikin yanki guda ta shekaru ko ajin zamantakewa na iya ayyana mabambantan hasashe daban-daban na ingancin rigakafin. [66]
Kara karantawa
[gyara sashe | gyara masomin]
- World Health Organization (2009). The immunological basis for immunization series : module 7: measles - Update 2009. Geneva, Switzerland: World Health Organization (WHO). hdl:10665/44038. ISBN 9789241597555.
- Ramsay M, ed. (2019). "Chapter 21: Measles". Immunisation against infectious disease. London, England: Public Health England. Archived from the original on 12 November 2019. Retrieved 22 December 2019.
- Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. (2021). "Chapter 13: Measles". Epidemiology and Prevention of Vaccine-Preventable Diseases (14th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 30 December 2016. Retrieved 22 December 2019.
- Gastanaduy PA, Redd SB, Clemmons NS, Lee Adria D, Hickman CJ, Rota PA, Patel M (2019). "Chapter 7: Measles". In Roush SW, Baldy LM, Hall MH (eds.). Manual for the surveillance of vaccine-preventable diseases. Atlanta, Georgia: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 1 August 2020. Retrieved 22 December 2019.
Hanyoyin haɗi na waje
[gyara sashe | gyara masomin]- "MMR (Measles, Mumps, & Rubella) Vaccine Information Statement". U.S. Centers for Disease Control and Prevention (CDC). 22 October 2019.
- "MMRV (Measles, Mumps, Rubella & Varicella) Vaccine Information Statement". U.S. Centers for Disease Control and Prevention (CDC). 22 October 2019.
- Measles Vaccine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Manazarta
[gyara sashe | gyara masomin]- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 World Health Organization (April 2017). "Measles vaccines: WHO position paper – April 2017" (PDF). Weekly Epidemiological Record. 92 (17): 205–27. PMID 28459148. Archived (PDF) from the original on 23 January 2023. Retrieved 23 September 2020. Cite error: Invalid
<ref>tag; name "WHO2017Vac" defined multiple times with different content - ↑ "Measles Vaccination and Infection: Questions and Misconceptions". ASM.org (in Turanci). Archived from the original on 3 April 2022. Retrieved 29 April 2022.
- ↑ "Summary of the WHO position on Measles Vaccine- April 2017" (PDF). who.int. 20 July 2017. Archived from the original (PDF) on 8 March 2022.
- ↑ "Measles". www.who.int (in Turanci). Archived from the original on 1 June 2019. Retrieved 29 April 2022.
- ↑ 5.0 5.1 5.2 "About Measles Vaccination | Vaccination and Immunizations | CDC". www.cdc.gov (in Turanci). 9 January 2020. Archived from the original on 27 April 2020. Retrieved 30 April 2020. Cite error: Invalid
<ref>tag; name "CDC2020Me" defined multiple times with different content - ↑ 6.0 6.1 CDC (2 August 2019). "Measles and the Vaccine (Shot)". Centers for Disease Control and Prevention (in Turanci). Archived from the original on 29 January 2020. Retrieved 30 April 2020.
- ↑ "Measles, Mumps, and Rubella (MMR) Vaccination | CDC". www.cdc.gov (in Turanci). 5 April 2021. Archived from the original on 26 April 2020. Retrieved 29 April 2022.
- ↑ Mitchell D (2013). The essential guide to children's vaccines. New York: St. Martin's Press. p. 127. ISBN 9781466827509. Archived from the original on 8 September 2017.
- ↑ 9.0 9.1 "ProQuad- measles, mumps, rubella and varicella virus vaccine live injection, powder, lyophilized, for suspension". DailyMed. 26 September 2019. Archived from the original on 6 April 2020. Retrieved 29 January 2020. Cite error: Invalid
<ref>tag; name "ProQuad label" defined multiple times with different content - ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 "MMR Vaccination | What You Should Know | Measles, Mumps, Rubella | CDC". www.cdc.gov (in Turanci). 24 December 2019. Archived from the original on 26 April 2020. Retrieved 30 April 2020. Cite error: Invalid
<ref>tag; name "www.cdc.gov_2019" defined multiple times with different content - ↑ "Information Sheet Observed Rate of Vaccine Reactions" (PDF). World Health Organization (WHO). Archived (PDF) from the original on 19 December 2019. Retrieved 1 December 2018.
- ↑ "MEASLES VACCINE - Essential drugs". medicalguidelines.msf.org. Archived from the original on 5 December 2021. Retrieved 28 April 2022.
- ↑ 13.0 13.1 13.2 CDC (5 November 2020). "History of Measles". Centers for Disease Control and Prevention (in Turanci). Archived from the original on 6 April 2020. Retrieved 9 February 2021. Cite error: Invalid
<ref>tag; name "CDC_2020" defined multiple times with different content - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedCDC2018Hist - ↑ "Vaccine History: Developments by Year". The Children's Hospital of Philadelphia. 20 November 2014. Archived from the original on 28 April 2022. Retrieved 28 April 2022.
- ↑ "Measles Fact Sheet - WHO". World Health Organization (WHO). 20 March 2023. Archived from the original on 28 November 2022. Retrieved 29 May 2023.
- ↑ "Immunization Coverage - WHO". World Health Organization (WHO). 14 July 2022. Archived from the original on 6 February 2018. Retrieved 29 May 2023.
- ↑ Empty citation (help)
- ↑ "Measles". www.who.int (in Turanci). Archived from the original on 1 June 2019. Retrieved 28 April 2022.
- ↑ Abramson B (2018). Vaccine, vaccination, and immunization law. Bloomberg Law. pp. 10–30. ISBN 9781682675830.
- ↑ Babbott FL, Gordon JE (September 1954). "Modern measles". The American Journal of the Medical Sciences. 228 (3): 334–61. doi:10.1097/00000441-195409000-00013. PMID 13197385.
- ↑ "Measles Prevention: Recommendations of the Immunization Practices Advisory Committee (ACIP)". www.cdc.gov. Archived from the original on 15 May 2012. Retrieved 27 April 2020.
- ↑ Centers for Disease Control and Prevention (CDC) (1994). "Summary of notifiable diseases, United States, 1993". MMWR. Morbidity and Mortality Weekly Report. 42 (53): i–xvii, 1–73. PMID 9247368. Archived from the original on 9 March 2010.
- ↑ Centers for Disease Control and Prevention (CDC) (July 2007). "Summary of notifiable diseases, United States, 2007". MMWR. Morbidity and Mortality Weekly Report. 56 (53): 1–94. Archived from the original on 13 June 2018. Retrieved 10 September 2017.
- ↑ Wallace G, Leroy Z (2015). "Measles". In Hamborsky J, Kroger A, Wolfe S (eds.). Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: Public Health Foundation. Archived from the original on 7 February 2015. Retrieved 30 April 2019.
- ↑ "Measles Cases and Outbreaks". Archived from the original on 13 February 2015. Retrieved 30 November 2018.
- ↑ Bloch AB, Orenstein WA, Stetler HC, Wassilak SG, Amler RW, Bart KJ, Kirby CD, Hinman AR (October 1985). "Health impact of measles vaccination in the United States". Pediatrics. 76 (4): 524–32. doi:10.1542/peds.76.4.524. PMID 3931045. S2CID 6512947.
- ↑ Centers for Disease Control and Prevention (CDC) (March 2006). "Progress in reducing global measles deaths, 1999-2004". MMWR. Morbidity and Mortality Weekly Report. 55 (9): 247–9. PMID 16528234. Archived from the original on 16 October 2007.
- ↑ 29.0 29.1 "Dr. Thomas C. Peebles, Who Identified Measles Virus, Dies at 89". The New York Times. 4 August 2010. Archived from the original on 6 April 2020. Retrieved 11 February 2017. Cite error: Invalid
<ref>tag; name "NYTPeebles" defined multiple times with different content - ↑ Collins H (30 August 1999). "The Man Who Saved Your Life - Maurice R. Hilleman - Developer of Vaccines for Mumps and Pandemic Flu". The Philadelphia Inquirer. Archived from the original on 6 March 2009. Retrieved 28 January 2020.
- ↑ "Measles". www.who.int (in Turanci). Retrieved 2024-08-21.
- ↑ 32.0 32.1 32.2 32.3 Parker AA, Staggs W, Dayan GH, Ortega-Sánchez IR, Rota PA, Lowe L, Boardman P, Teclaw R, Graves C, LeBaron CW (August 2006). "Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States". The New England Journal of Medicine. 355 (5): 447–55. doi:10.1056/NEJMoa060775. PMID 16885548.
- ↑ Centers for Disease Control and Prevention (CDC) (December 2006). "Measles--United States, 2005". MMWR. Morbidity and Mortality Weekly Report. 55 (50): 1348–51. PMID 17183226. Archived from the original on 13 March 2015.
- ↑ Mina MJ (June 2017). "Measles, immune suppression and vaccination: direct and indirect nonspecific vaccine benefits". The Journal of Infection. 74 (Suppl 1): S10–S17. doi:10.1016/S0163-4453(17)30185-8. PMID 28646947.
- ↑ Nic Lochlainn LM, de Gier B, van der Maas N, van Binnendijk R, Strebel PM, Goodman T, de Melker HE, Moss WJ, Hahné SJ (November 2019). "Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: a systematic review and meta-analysis". The Lancet. Infectious Diseases (in English). 19 (11): 1246–1254. doi:10.1016/S1473-3099(19)30396-2. PMC 6838663. PMID 31548081.CS1 maint: unrecognized language (link)
- ↑ Sankoh O, Welaga P, Debpuur C, Zandoh C, Gyaase S, Poma MA, Mutua MK, Hanifi SM, Martins C, Nebie E, Kagoné M, Emina JB, Aaby P (June 2014). "The non-specific effects of vaccines and other childhood interventions: the contribution of INDEPTH Health and Demographic Surveillance Systems". International Journal of Epidemiology. 43 (3): 645–653. doi:10.1093/ije/dyu101. PMC 4052142. PMID 24920644.
- ↑ Natukunda A, Zirimenya L, Nassuuna J, Nkurunungi G, Cose S, Elliott AM, Webb EL (September 2022). "The effect of helminth infection on vaccine responses in humans and animal models: A systematic review and meta-analysis". Parasite Immunology. 44 (9): e12939. doi:10.1111/pim.12939. PMC 9542036 Check
|pmc=value (help). PMID 35712983 Check|pmid=value (help). - ↑ "Vaccine (Shot) for Measles". Centers for Disease Control and Prevention (CDC). 2 August 2019. Archived from the original on 29 January 2020. Retrieved 29 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Birth-18 Years Immunization Schedule". Centers for Disease Control and Prevention (CDC). 5 February 2019. Archived from the original on 6 March 2016. Retrieved 29 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Birth-18 Years Immunization Schedule". Centers for Disease Control and Prevention (CDC). 5 February 2019. Archived from the original on 6 March 2016. Retrieved 29 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Measles". National Health Service UK. 20 October 2017. Archived from the original on 8 March 2019. Retrieved 11 March 2019.
- ↑ "MMR vaccine overview". National Health Service UK. 8 October 2019. Archived from the original on 31 January 2013. Retrieved 29 January 2020.
- ↑ "Measles vaccine: Canadian immunization guide". Public Health Agency of Canada. 18 July 2007. Archived from the original on 17 March 2019. Retrieved 13 March 2019.
- ↑ "Information Sheet: Observed Rate of Vaccine Reactions: Measles, Mumps and Rubella Vaccines" (PDF). World Health Organization (WHO). May 2014. Archived (PDF) from the original on 17 December 2019. Retrieved 1 December 2018.
- ↑ 45.0 45.1 Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V (November 2021). "Vaccines for measles, mumps, rubella, and varicella in children". The Cochrane Database of Systematic Reviews. 2021 (11): CD004407. doi:10.1002/14651858.CD004407.pub5. PMC 8607336 Check
|pmc=value (help). PMID 34806766 Check|pmid=value (help). - ↑ "Measles, mumps, and rubella (MMR) vaccine". Centers for Disease Control and Prevention. 22 August 2008. Archived from the original on 8 October 2008. Retrieved 21 December 2008.
- ↑ Institute of Medicine (US) Immunization Safety Review Committee (17 May 2004). Immunization Safety Review: Vaccines and Autism. Institute of Medicine of the National Academy of Sciences. doi:10.17226/10997. ISBN 978-0-309-09237-1. PMID 20669467. Archived from the original on 7 October 2014. Retrieved 19 October 2019.
- ↑ "Guidelines for Vaccinating Pregnant Women". Centers for Disease Control and Prevention. August 2016. Archived from the original on 6 April 2020. Retrieved 30 April 2019.
- ↑ "Contraindications and Precautions". Vaccine Recommendations and Guidelines of the ACIP. Centers for Disease Control and Prevention. 23 April 2020. Archived from the original on 1 May 2019. Retrieved 30 April 2019.
- ↑ "Work by Enders Brings Measles Vaccine License". The Hartford Courant. 22 March 1963. Archived from the original on 3 November 2012.
A strain of measles virus isolated in 1954 by Dr. Thomas C. Peebles, instructor in pediatrics at Harvard, and Enders, formed the basis for the development of the present vaccine
- ↑ Griffin DE (March 2018). "Measles Vaccine". Viral Immunol. 31 (2): 86–95. doi:10.1089/vim.2017.0143. PMC 5863094. PMID 29256824.
- ↑ "The Measles Vaccine". The New York Times. 28 March 1963. Archived from the original on 30 April 2019. Retrieved 30 April 2019.
- ↑ Hilleman MR (July 1992). "Past, present, and future of measles, mumps, and rubella virus vaccines". Pediatrics. 90 (1 Pt 2): 149–53. doi:10.1542/peds.90.1.149. PMID 1603640. S2CID 33115842.
- ↑ Beautysays (27 September 2009). "David Morley - a career of service that started in Nigeria". Nigeria Health Watch (in Turanci). Archived from the original on 29 June 2022. Retrieved 29 April 2022.
- ↑ Morley DC, Woodland M, Krugman S, Friedman H, Grab B (1964). "Measles and Measles Vaccination in an African Village". Bulletin of the World Health Organization. 30 (5): 733–9. PMC 2554995. PMID 14196817.
- ↑ Pritchard J (13 November 1997). "Obituary: Dr C. A. Pearson". The Independent. Archived from the original on 24 February 2014. Retrieved 29 January 2014.
- ↑ Ovsyannikova IG, Johnson KL, Naylor S, Poland GA (February 2005). "Identification of HLA-DRB1-bound self-peptides following measles virus infection". Journal of Immunological Methods. 297 (1–2): 153–67. doi:10.1016/j.jim.2004.12.020. PMID 15777939.
- ↑ 58.0 58.1 58.2 "M-M-R II- measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension". DailyMed. 24 September 2019. Archived from the original on 6 April 2020. Retrieved 29 January 2020. Cite error: Invalid
<ref>tag; name "package insert" defined multiple times with different content - ↑ "Q&As about Monovalent M-M-R Vaccines". Centers for Disease Control and Prevention (CDC). 26 October 2009. Archived from the original on 19 October 2019. Retrieved 18 October 2019.
- ↑ Atwood A (2022). "The Long-Term Effects of Measles Vaccination on Earnings and Employment". American Economic Journal: Economic Policy (in Turanci). 14 (2): 34–60. doi:10.1257/pol.20190509. ISSN 1945-7731. S2CID 248468606 Check
|s2cid=value (help). - ↑ "M-M-RVaxPro EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 6 April 2020. Retrieved 29 January 2020.
- ↑ "Priorix - Summary of Product Characteristics (SmPC)". (emc). 14 January 2020. Archived from the original on 6 April 2020. Retrieved 29 January 2020.
- ↑ "VFC | Home | Vaccines for Children Program | CDC". www.cdc.gov (in Turanci). 2 April 2019. Archived from the original on 1 May 2020. Retrieved 30 April 2020.
- ↑ "State Vaccination Requirements | CDC". www.cdc.gov (in Turanci). 11 March 2019. Archived from the original on 2 April 2020. Retrieved 30 April 2020.
- ↑ "MMR Vaccine Mandates for Child Care and K-12". www.immunize.org. Archived from the original on 12 June 2020. Retrieved 30 April 2020.
- ↑ Scirè G (2021). "Modelling and assessing public health policies to counteract Italian measles outbreaks". International Journal of Simulation and Process Modelling (in Turanci). 16 (4): 271. doi:10.1504/IJSPM.2021.118832. ISSN 1740-2123.
|hdl-access=requires|hdl=(help)
- Pages with reference errors
- CS1 Turanci-language sources (en)
- Pages with empty citations
- CS1 maint: unrecognized language
- CS1 errors: PMC
- CS1 errors: PMID
- CS1 errors: S2CID
- CS1 errors: param-access
- Articles using generic infobox
- All articles with unsourced statements
- Articles with unsourced statements from June 2022
- Articles with invalid date parameter in template
- Rigakafi
- Shafuka masu fassarorin da ba'a duba ba
